A new human skin atlas and skin organoid could revolutionize treatments for skin disorders and enhance regenerative medicine techniques.
A team of researchers has created the first single-cell atlas of prenatal human skin to understand how skin forms, and what goes wrong in disease.
The team, comprised of researchers from the Wellcome Sanger Institute, Newcastle University, and their collaborators, used single-cell sequencing and other genomics techniques to create the atlas and uncover how human skin, including hair follicles, is formed. These findings could be used to create new hair follicles in regenerative medicine and skin transplants for burn victims.
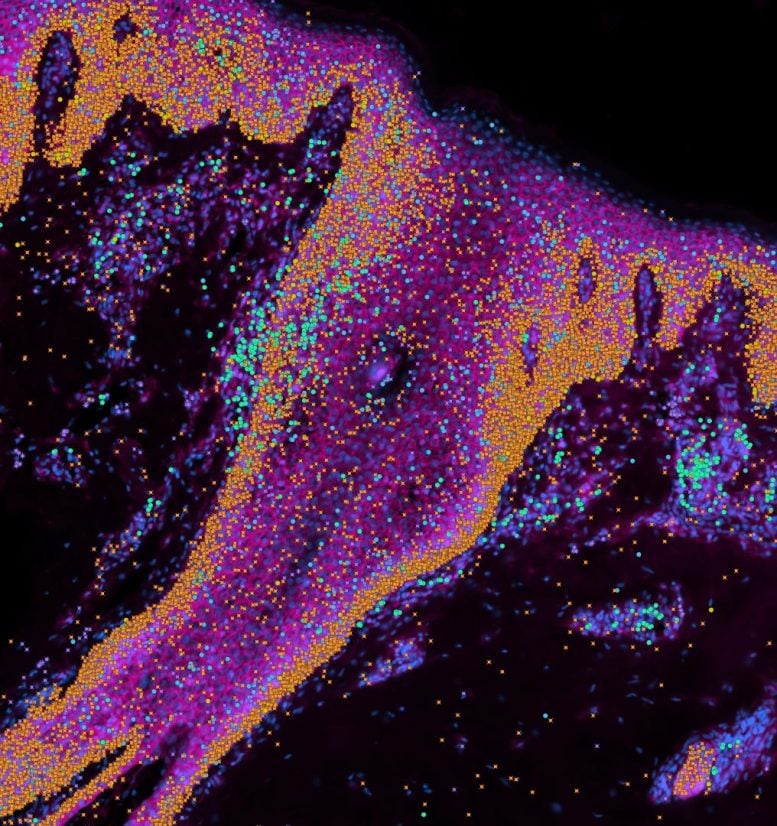
Development and Application of a Skin Organoid
In the study, recently published in Nature, the team also created a ‘mini-organ’ of skin in a dish with the ability to grow hair. Using the organoid, they showed how immune cells play an important role in scarless skin repair, which could lead to clinical applications to prevent scarring after surgery, or scarless healing after wounding.
As part of the Human Cell Atlas,[1] which is mapping all cell types in the human body to transform understanding of health and disease, the researchers provide a molecular ‘recipe’ to build skin and a new organoid model to study congenital skin diseases.
Skin is the largest organ of the human body, measuring on average two square meters. It provides a protective barrier, regulates our body temperature, and can regenerate itself. Skin develops in the sterile environment of the womb, with all hair follicles formed before birth – there is follicle cycling after birth, but no new follicles are made. Before birth, skin has the unique ability to heal without scarring.
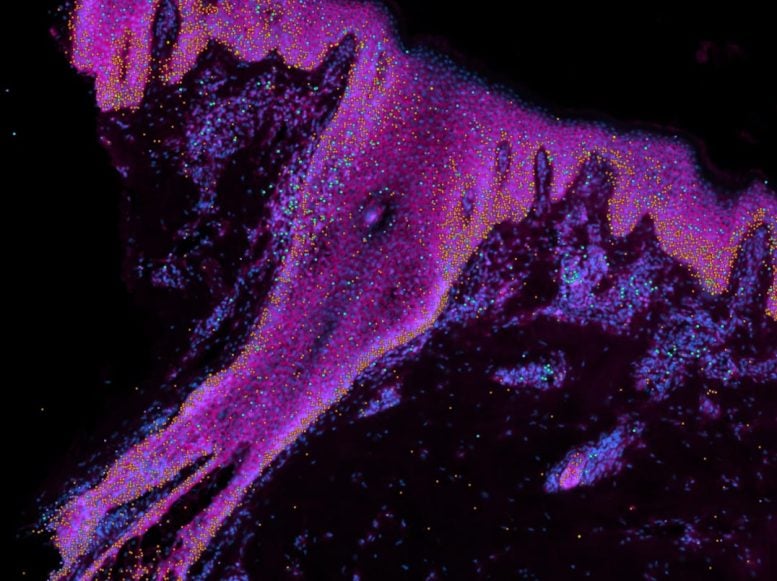
Skin Development and Genetics Research
It has been difficult to study how the human skin develops, as animal models have key differences. As part of the Human Cell Atlas, a team of researchers is focused on learning how human skin is built. Understanding how skin develops, where cells are in space and time, and the role of genetics will help reveal how specific mutations cause congenital skin disorders, such as blistering disorders and scaly skin.
In this new study, researchers at the Wellcome Sanger Institute, Newcastle University, and their collaborators created the first single-cell and spatial atlas of human prenatal skin.
The team used samples of prenatal skin tissue,[2] which they broke down to look at individual cells in suspension, as well as cells in place within the tissue. Scientists used cutting-edge single-cell sequencing and spatial transcriptomics[3] to analyze individual cells in space and time, and the cellular changes that regulate skin and hair follicle development. They described the steps that outline how human hair follicles are formed and identified differences from mouse hair follicles.

Advancements in Skin Organoid Models
Using adult stem cells,[4] the researchers also created a ‘mini-organ’ of skin in a dish, known as an organoid, with the ability to grow hair. They compared the molecular characteristics of skin organoids with prenatal skin and found the skin organoid model more closely resembled prenatal skin than adult skin.
The team found that blood vessels did not form in the skin organoid as well as prenatal skin. By adding immune cells known as macrophages to the organoid, they discovered the macrophages promoted the formation of blood vessels, and the team undertook 3D imaging to assess blood vessel formation within the tissue.
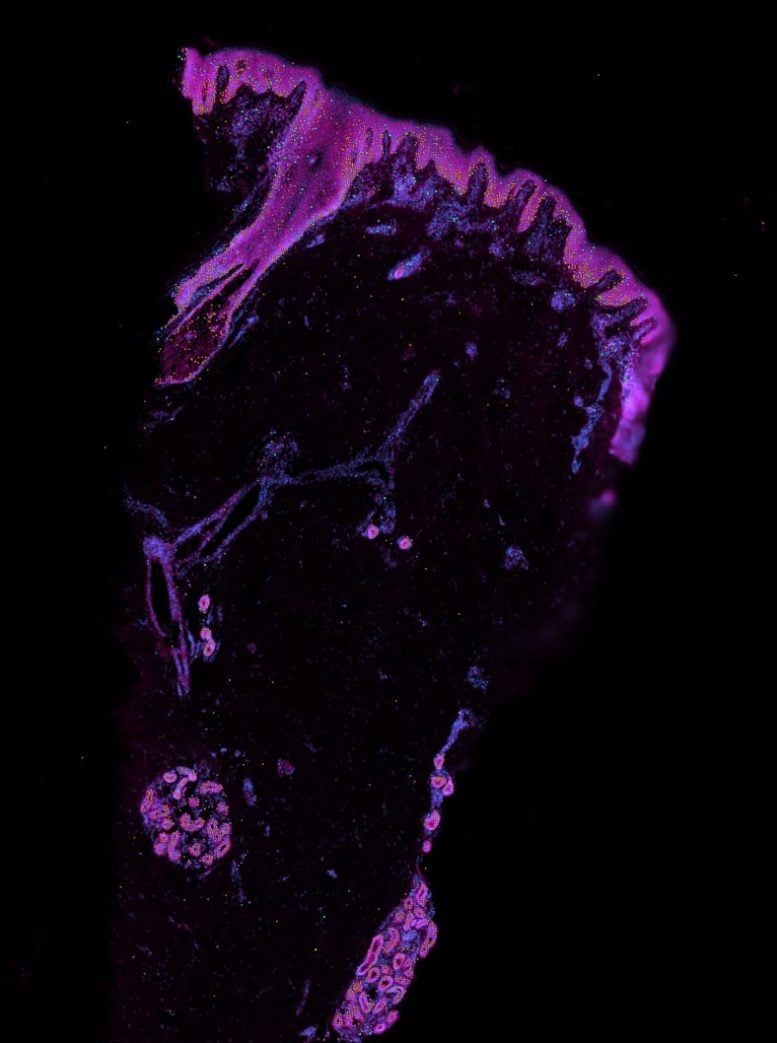
Role of Macrophages in Skin Development
It’s known that these immune cells protect the skin from infection. However, this is the first time that macrophages have been shown to play a key role in the formation of human skin during early development by supporting the growth of blood vessels. This offers an option to improve the vascularisation of other tissue organoids.
The team also analyzed differences in cell types between prenatal skin and adult skin. They show how macrophages play an important role in scarless skin repair in prenatal skin, which could lead to clinical applications to avoid scarring after surgery or wounding.

Clinical Applications and Future Research
As a result of this study, the team provides a molecular ‘recipe’ for how human skin is built and how hair follicles form. These insights could be used in the creation of new hair follicles for regenerative medicine, such as for skin transplants for burn victims, or those with scarring alopecia.
The prenatal human skin atlas will also be used to identify in which cells the genes are active, or expressed, that are known to cause congenital hair and skin disorders, such as blistering disorders and scaly skin. The researchers found that genes involved in these disorders are expressed in prenatal skin, meaning they originate in utero. The skin organoids created in this study offer a new, accurate model for studying these diseases.
“With our prenatal human skin atlas, we’ve provided the first molecular ‘recipe’ for making human skin and uncovered how human hair follicles are formed before birth. These insights have amazing clinical potential and could be used in regenerative medicine, when offering skin and hair transplants, such as for burn victims or those with scarring alopecia,” said Dr Elena Winheim, co-first author from the Wellcome Sanger Institute.
Dr Hudaa Gopee, co-first author from Newcastle University, said: “We’re excited to have made a skin organoid model that grows hair. In this process, we uncovered a new, important role of immune cells in promoting the growth of blood vessels in developing skin tissue, which could help improve other organoid models. These immune cells, called macrophages, also appear to play a key part in scarless skin repair in prenatal skin. Our findings could inform clinical advances to avoid scarring after surgery.”
Professor Muzlifah Haniffa, co-lead author and Interim Head of Cellular Genetics at the Wellcome Sanger Institute, said: “Our prenatal human skin atlas and organoid model provide the research community with freely available tools to study congenital skin diseases and explore regenerative medicine possibilities. We are making exciting strides towards creating the Human Cell Atlas, understanding the biological steps of how humans are built, and investigating what goes wrong in disease.”
Notes
- This study is part of the Human Cell Atlas (HCA), an international collaborative consortium which is creating comprehensive reference maps of all human cells — the fundamental units of life — as a basis for understanding human health and for diagnosing, monitoring, and treating disease. The HCA is likely to impact every aspect of biology and medicine, propelling translational discoveries and applications and ultimately leading to a new era of precision medicine. The HCA was co-founded in 2016 by Dr Sarah Teichmann, then at the Wellcome Sanger Institute (UK) and Dr Aviv Regev, then at the Broad Institute of MIT and Harvard (USA). A truly global initiative, there are now more than 3,500 HCA members, from 101 countries around the world. https://www.humancellatlas.org
- Prenatal skin tissue samples were provided by the Human Developmental Biology Resource (HDBR) and the Cambridge Bio Resource.
- Spatial transcriptomics is a molecular method that maps gene activity in a tissue sample, and its location. It allows researchers to accurately measure gene expression at the cellular level within intact tissue samples.
- The skin organoid was produced using induced pluripotent stem cells (iPSCs) from the KOLF cell line, generated by the Human Induced Pluripotent Stem Cell Initiative (HipSci). Find out more about HipSci: https://www.hipsci.org/
Reference: “A prenatal skin atlas reveals immune regulation of human skin morphogenesis” by Nusayhah Hudaa Gopee, Elena Winheim, Bayanne Olabi, Chloe Admane, April Rose Foster, Ni Huang, Rachel A. Botting, Fereshteh Torabi, Dinithi Sumanaweera, Anh Phuong Le, Jin Kim, Luca Verger, Emily Stephenson, Diana Adão, Clarisse Ganier, Kelly Y. Gim, Sara A. Serdy, CiCi Deakin, Issac Goh, Lloyd Steele, Karl Annusver, Mohi-Uddin Miah, Win Min Tun, Pejvak Moghimi, Kwasi Amoako Kwakwa, Tong Li, Daniela Basurto Lozada, Ben Rumney, Catherine L. Tudor, Kenny Roberts, Nana-Jane Chipampe, Keval Sidhpura, Justin Englebert, Laura Jardine, Gary Reynolds, Antony Rose, Vicky Rowe, Sophie Pritchard, Ilaria Mulas, James Fletcher, Dorin-Mirel Popescu, Elizabeth Poyner, Anna Dubois, Alyson Guy, Andrew Filby, Steven Lisgo, Roger A. Barker, Ian A. Glass, Jong-Eun Park, Roser Vento-Tormo, Marina Tsvetomilova Nikolova, Peng He, John E. G. Lawrence, Josh Moore, Stephane Ballereau, Christine B. Hale, Vijaya Shanmugiah, David Horsfall, Neil Rajan, John A. McGrath, Edel A. O’Toole, Barbara Treutlein, Omer Bayraktar, Maria Kasper, Fränze Progatzky, Pavel Mazin, Jiyoon Lee, Laure Gambardella, Karl R. Koehler, Sarah A. Teichmann and Muzlifah Haniffa, 16 October 2024, Nature.
DOI: 10.1038/s41586-024-08002-x
Funding: This work was supported by Wellcome, the CIFAR MacMillan Multiscale Human program, and others. Full funding details are available in the publication.
This post was originally published on here







