Ice cream has a simple ingredient list – just cream and sugar, but as anyone who’s tried to make it knows, this frozen dessert can easily become a sticky, icy mess. Food scientist Douglas Goff explains how lessons from materials science can teach us how to make the perfect scoop
What exactly is ice cream? For most of us, it’s a tasty frozen dessert, but to food scientists like Douglas Goff, it’s also a marvel of physics and chemistry. Ice cream is a complex multiphase material, containing emulsion, foam, crystals, solutes and solvent. Whether made in a domestic kitchen or on a commercial scale, ice cream requires a finely tuned ratio of ingredients and precision control during mixing, churning and freezing.
Goff is a researcher in food science at the University of Guelph in Canada and an expert in the science of ice cream. In addition to his research studying, among other things, structure and ingredient functionality in ice cream, Goff is also the instructor on the University of Guelph’s annual ice-cream course, which, having been taught since 1914, is the longest-running at the university.
In a conversation with Physics World’s Hamish Johnston, Goff explains the science of ice cream, why it’s so hard to make vegan ice cream and how his team performs electron microscopy experiments without their samples melting.
How would you describe the material properties of ice cream to a physicist?
Ice cream is an incredibly complex multi-phase system. It starts as an emulsion, where fat droplets are dispersed in a sugary water-based solution. Then we whip the emulsion to incorporate an air phase into it – this is called foaming (see “Phases in ice cream”). In a frozen tub of ice cream, about half of the volume is air. That air is present in the form of tiny bubbles that are distributed throughout the product.
Then we partially freeze the aqueous phase, turning at least half of the water into microscopically small ice crystals. The remaining unfrozen phase is what makes the ice cream soft, scoopable and chewable. It remains unfrozen because of all the sugar that’s dissolved in it, which depresses the freezing point.
So you end up with fat droplets in the form of an emulsion, air bubbles in the form of a foam, a partially crystalline solvent in the form of ice crystals, and a concentrated sugar solution.
Phases in ice cream

Emulsion: Some liquids, such as oil and water, will not mix if a droplet of one is added to the other – they are said to be immiscible. If many droplets of one liquid can be stabilized in another without coalescing, the resulting mixture is called an emulsion (left image).
Foam: A foam, like an emulsion, consists of two phases where one is dispersed in the other. In the case of foam, many tiny gas bubbles are trapped in a liquid or solid (right image).
Glass: When a liquid is cooled below a certain temperature, it generally undergoes a first-order phase transition to a solid crystal. However, if a liquid can be cooled below its freezing point without crystallizing (supercooling) – for example, if it is cooled very quickly, it may form glass – an amorphous solid with a disordered, liquid-like structure but solid-like mechanical properties. The temperature at which the glass forms, marked by a rapid increase in the material’s viscosity, is called the glass transition temperature.
What are the length scales of the different phases in the ice cream?
We’ve done a lot of electron microscopy research studying this in my lab. In fact, our research was some of the very first that utilized electron microscopy techniques for the structure of ice cream. The fat droplets are about one micron in diameter and the air bubbles, depending on the equipment that’s used, would be about 20 to 30 microns in diameter. The ice crystals are in the 10 to 20 micron size range.
It really is a beautiful thing to look at under an electron microscope, depending on the technique that you use (see image).
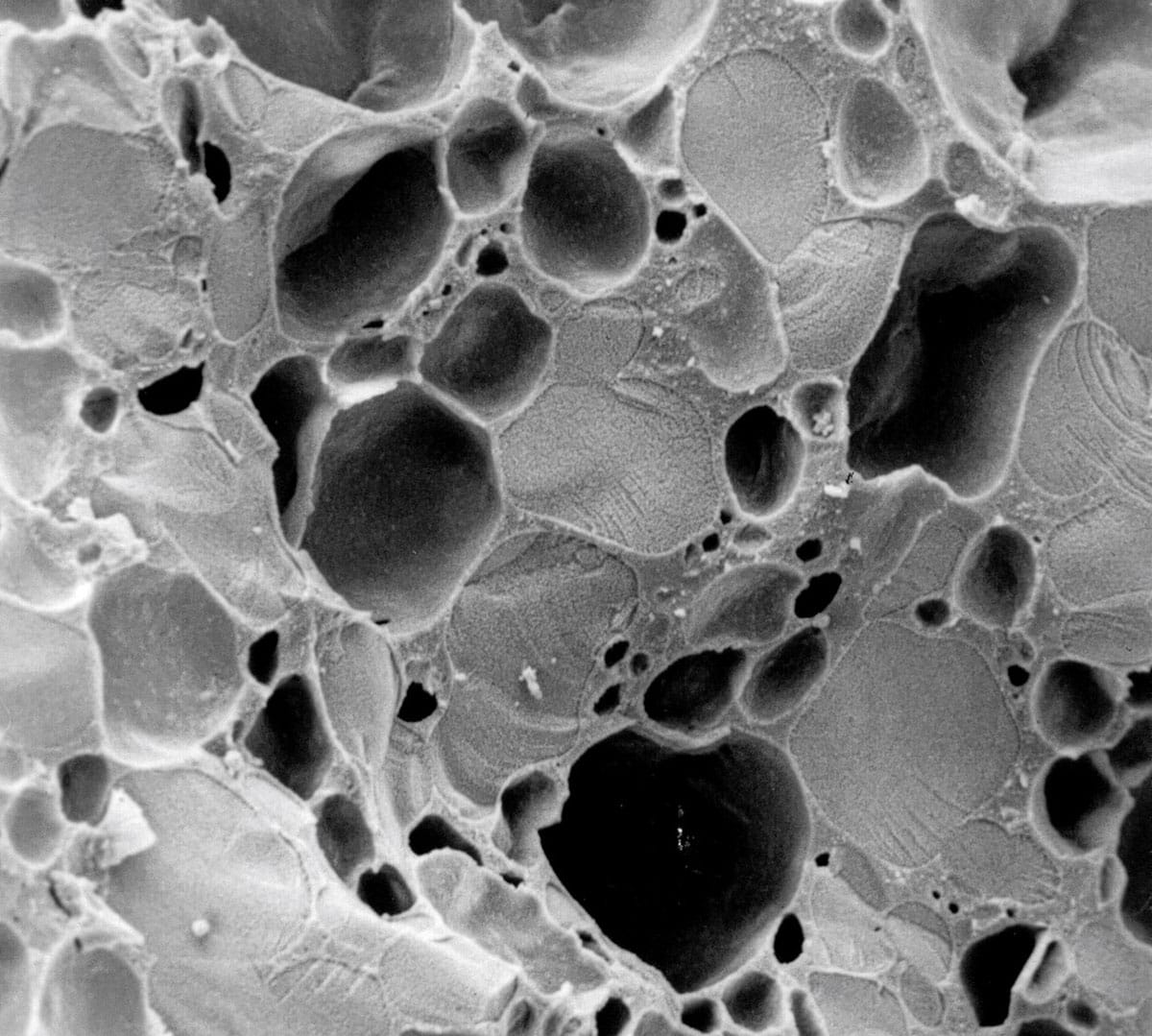
What are the big differences between ice cream that’s made in a commercial setting versus a domestic kitchen?
The freezing and whipping happen at the same time whether it’s an ice cream maker in the kitchen or a commercial operation. The biggest difference between what you do in the kitchen and what they’re going to do in the factory is the structure of the ice cream. Homemade ice cream is fine for maybe a day or two, but it starts to get icy pretty quickly, whereas we want a shelf life of months to a year when ice cream is made commercially.
This is because of the way the ice phase evolves over time – a process called recrystallization. If ice cream warms up it starts to melt. When the temperature is lowered again, water is frozen back into the ice phase, but it doesn’t create new ice crystals, it just grows onto the existing ice crystals.
This means that if ice cream is subject to lots of temperature fluctuation during storage, it’s going to degrade and become icy much quicker than if it was stored at a constant temperature. The warmer the temperature, the faster the rate of recrystallization. Commercial freezing equipment will give you much smaller ice crystal size than homemade ice cream machines. Low and constant temperature storage is what everybody strives for, and so the lower the temperature and the more constant it is, and the smaller the ice crystals are to begin with, the longer your shelf life before changes start occurring.
There’s also another structural element that is important for the long-term storage of ice cream. When that unfrozen sugary solvent phase gets concentrated enough, it can undergo a glass transition (see “Phases in ice cream”). Glass is an amorphous solid, so if this happens, there will be no movement of water or solute within the system and it can remain unchanged for years. For ice cream, the glass transition temperature is around –28 to –32° C so if you want long-term storage, you have to get down below that that glass transition temperature.
The third thing is the addition of stabilisers. Those are things like locust bean gum, guar gum or cellulose gum and there are some novel ones as well. What those do is increase the viscosity in the unfrozen phase. This slows down the rate of ice recrystallization because it slows down the diffusion of water and the growth of ice.
There are also some other novel agents that can prevent ice from recrystallizing into large crystals. One of these is called propylene glycol monostearate, it absorbs onto the surface of an ice crystal and prevents it from growing as the temperature fluctuates. This is also something we see in nature. Some insect, fish and plant species that live in cold environments have proteins that control the growth of ice in their blood and tissues. A lot of fish, for example, swim around with minute ice crystals in their in their body, but the proteins prevent the crystals from getting big enough to cause harm.

How does adding flavourings to ice cream change the manufacturing process?
When you think about ice cream around the world, there are hundreds of different flavours. The important question is whether the flavouring will impact the solution or emulsion.
For example, a chocolate chip will be inert, it’s not going to interact at all with the rest of the matrix. Strawberries on the other hand, really impact the system because of the high sugar content in the fruit preparation. We need to add sugar to the fruit to make sure it is softer than the ice cream itself – you don’t want to bite into ice cream and find a hard, frozen berry. The problem is that some of that sugar will diffuse into the unfrozen phase and lower its freezing point. This means that if you don’t do anything to the formulation, strawberry ice cream will be softer than something like vanilla because of the added sugar.
Another example would be alcohol-based flavours, anything from rum to Baileys Irish Cream or Frangelico, or even wine and beer. They’re very popular but the alcohol depresses the freezing point, so if you add enough to give you the flavour intensity that you want, your product won’t freeze. In that case, you might need to add less of the alcohol and a little bit more of a de-alcoholized flavouring.
You can try to make ice cream with just about any flavour, but you certainly have to look at what that flavouring is going to do to the structure and things like shelf life and so on.

Nowadays one can also buy vegan ice creams. How do the preparation and ingredients differ compared to dairy products?
A lot of it will be similar. We’re going to have an emulsified fat source, typically something like coconut oil or palm kernel oil, and then there’s the sugar, stabilisers and so on that you would have in a dairy ice cream.
The difference is the protein. Milk protein is both a very good foaming agent and a very good emulsifying agent. [Emulsifying and foaming agents are molecules that stabilize foams and emulsions. The molecules attach to the surface of the liquid droplets or air bubbles and stop them from coalescing with each other.] Plant proteins aren’t very good at either. If you look at cashew, almond or soy-based products, you’ll find additional ingredients to deliver the functionality that we would otherwise get from the milk protein.
What techniques do you use to study ice cream? And how do you stop the ice cream from melting during an experiment?
The workhorses of instrumentation for research are particle size analysis, electron microscopy and rheology (see “Experimental techniques”).
So first there’s laser light scattering which tells us everything we need to know about the fat globules and fat structure (see “Experimental techniques”). Then we use a lot of optical microscopy. You either need to put the microscope in a freezer or cold box or have a cold stage where you have the ice cream on a slide inside a chamber that’s cooled with liquid nitrogen. On the electron microscopy side (see “Experimental techniques”), we’ve done a lot of cryo-scanning electron microscopy (SEM), with a low-temperature unit.
We’ve also done a lot of transmission electron microscopy (TEM), which generally uses a different approach. Instead of performing the experiment in cold conditions, we use a chemical that “fixes” the structure in place and then we dry it, typically using a technique called “critical point drying” (see “Experimental techniques”). It’s then sliced into thin samples and studied with the TEM.
Experimental techniques

Rheology: Rheology is the study of the flow and deformation of materials. A rheometer is an apparatus used to measure the response of different materials to applied forces.
Dynamic light scattering (DLS): A laser-based technique used to measure the size distribution of dispersed particles. Dispersed particles such as fat globules in ice cream exhibit Brownian motion, with small particles moving faster than larger particles. The interference of laser light scattered from the particles is used to calculate the characteristic timescale of the Brownian motion and the particle size distribution.
Electron microscopy: Imaging techniques that use a beam of electrons, rather than photons, to image a sample. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) are two common examples. SEM uses reflected electrons to study the sample surface, whereas TEM uses electrons travelling through a sample to understand its internal structure.
Critical point drying: When a sample is dried in preparation for microscopy experiments, the effects of surface tension between the water in the sample and the surrounding air can cause damage. At the critical point, the liquid and gas phases are indistinguishable, if the water in the sample is at its critical point during dehydration, there is no boundary between the water and vapour, and this protects the structure of the sample.
After decades of studying ice cream, do you still get excited about it?
Oh, absolutely. I’ve been fortunate enough to have travelled to many, many interesting countries and I always see what the ice cream market looks like when I’m there. It’s not just a professional thing. I also like to know what’s going on around the world so I can share that with people. But of course, how can you go wrong with ice cream? It’s such a fun product to be associated with.
This post was originally published on here







