This post was originally published on here
TIT Correspondent
[email protected]
Researchers have developed a sunlight-driven approach to break carbon–fluorine (C–F) bonds—among the strongest and most difficult chemical bonds to cleave—opening new possibilities for applications in pharmaceuticals, agrochemicals, recycling, and chemical manufacturing.
Fluorinated organic compounds are widely used across industries because they are readily available and easy to synthesize. However, converting poly- and perfluorinated compounds into more valuable derivatives requires activation of the extremely stable C–F bond, a long-standing challenge in chemistry. Conventional methods typically depend on harsh reaction conditions, expensive metal catalysts, and high energy input, making them less sustainable. In contrast, photocatalysis using sunlight offers a cleaner, energy-efficient alternative under mild and recyclable conditions.
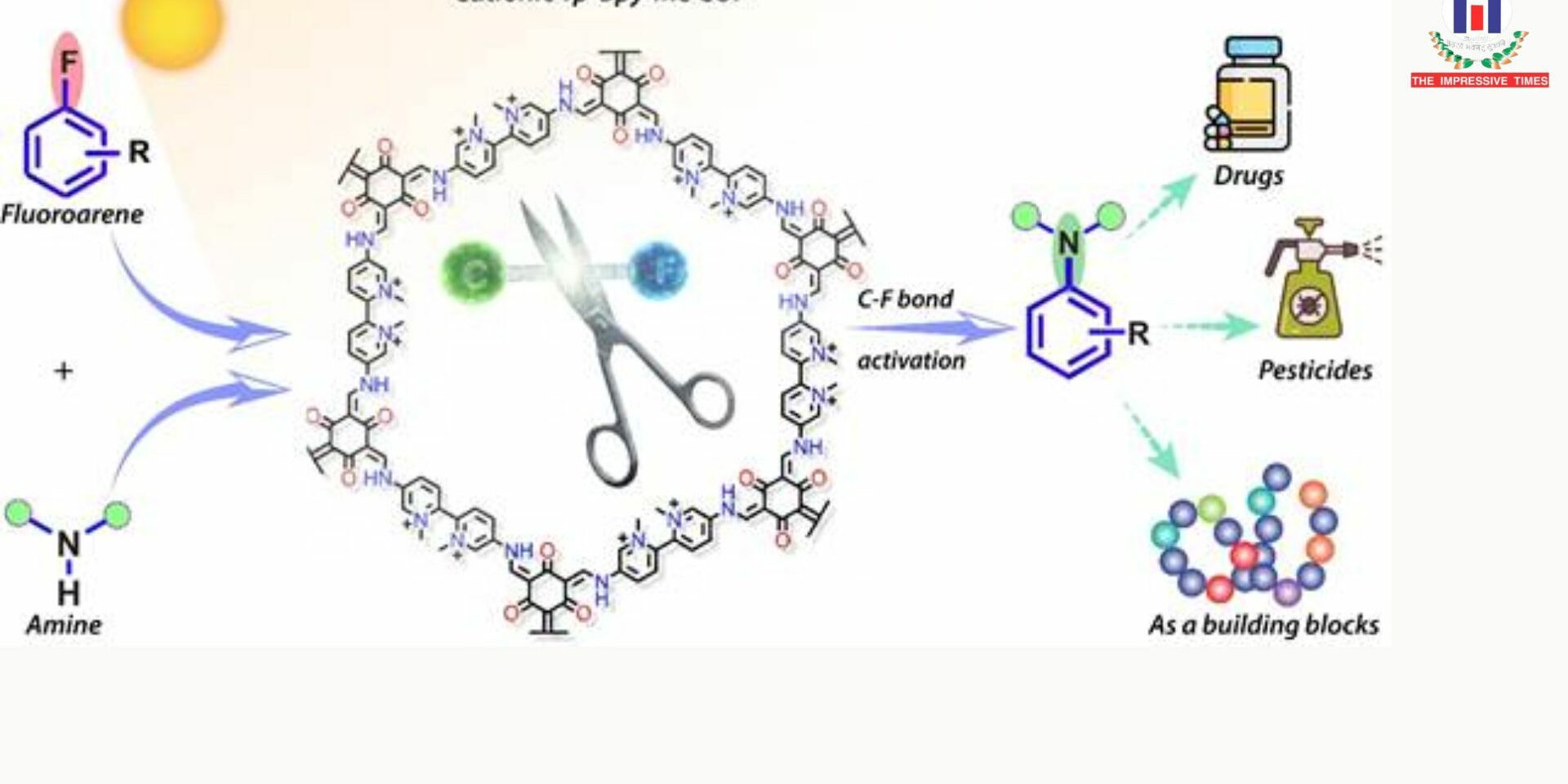
Scientists at the S. N. Bose National Centre for Basic Sciences, an autonomous institute under the Department of Science and Technology (DST), explored advanced porous materials known as covalent organic frameworks (COFs) to address this challenge. COFs are crystalline materials valued for their structural stability, large surface areas, and highly customizable architectures, which make them well suited for catalytic applications.
The research team engineered a bipyridine-based COF (Tp-Bpy) and introduced a simple post-synthetic modification by adding a methyl group. This step transformed the material into a positively charged variant, referred to as cationic Tp-Bpy-Me COF, without compromising the framework’s structure. The modification significantly enhanced the material’s electronic properties, allowing it to absorb visible light more effectively and facilitate demanding photocatalytic reactions.
Under blue light irradiation, the modified COF successfully activated strong C–F bonds and enabled the formation of carbon–nitrogen (C–N) bonds through reactions with amine nucleophiles—an essential step in constructing complex organic molecules. Notably, the process was also effective under natural sunlight, underscoring its potential as an environmentally sustainable method.
This study marks the first demonstration of a COF-based photocatalyst capable of converting C–F bonds into C–N bonds. The resulting compounds have promising applications in pharmaceutical and agrochemical development, as well as in the synthesis of diverse bioactive molecules.