This post was originally published on here
A hidden “jack-in-the-box” mechanism inside T cells may hold the key to unlocking more powerful cancer immunotherapies.
Over the last decade, one of the biggest leaps in cancer care has been T cell immunotherapy, which trains a person’s own immune system to spot and destroy harmful cells. Even so, scientists have not fully pinned down the exact steps that make these treatments work. That gap matters because T cell immunotherapies can be remarkably effective in certain cancer subtypes, but they fail for most cancers, and the reasons remain uncertain. A clearer picture of their modus operandi could help extend these benefits to many more patients.
A new look at the T cell receptor (TCR)
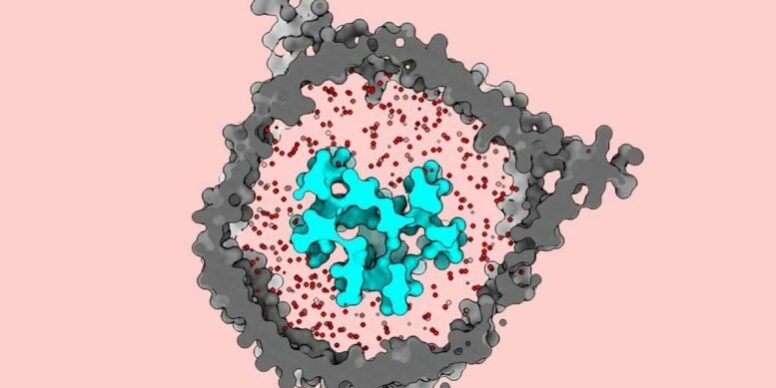
Researchers at The Rockefeller University have now uncovered important details about the T cell receptor (TCR), a key protein complex embedded in the cell membrane and central to T cell therapies. Using cryo-EM, the team from the Laboratory of Molecular Electron Microscopy imaged the receptor in a biochemical setup designed to mimic its native milieu. They found that the TCR behaves like a jack-in-the-box that snaps open when it encounters an antigen or another suspicious particle. This view runs counter to what earlier cryo-EM studies of the complex had suggested.
The results, published today (December 16) in Nature Communications, point to new ways to refine and broaden T cell therapies.
“This new fundamental understanding of how the signaling system works may help re-engineer that next generation of treatments,” says first author Ryan Notti, an instructor in clinical investigation in Walz’s lab and a special fellow in the Department of Medicine at Memorial Sloan Kettering Cancer Center, where he treats patients with sarcomas, or cancers that arise in soft tissue or bone.
“The T cell receptor is really the basis of virtually all oncological immunotherapies, so it’s remarkable that we use the system but really have had no idea how it actually works—and that’s where basic science steps in,” says Walz, a world expert in cryo-EM imaging. “This is some of the most important work to ever come out of my lab.”
How T cells recognize antigens and respond
Walz’s lab focuses on capturing detailed views of macromolecular complexes, especially proteins in cell membranes that coordinate communication between the outside and inside of a cell. The TCR is one of these complexes. Made of multiple proteins, it enables T cells to detect antigens displayed by human leukocyte antigen (HLA) complexes on other cells. T cell therapies rely on this natural detection system to rally the immune response against cancer.
Although researchers have known the building blocks of the TCR for decades, the earliest moments of activation have remained a mystery. Notti, who treats sarcoma patients as a physician-scientist, found that frustrating because many of his patients were not benefiting from T cell immunotherapies, and he wanted to understand why.
“Determining that would help us understand how the information gets from outside the cell, where those antigens are being presented by HLAs, to the inside of the cell, where signaling turns on the T cell,” he says.
Notti, who earned his Ph.D. in structural microbiology at Rockefeller before moving into oncology, suggested to Walz that they take a closer look at the problem together.
Nanodiscs and custom membranes reveal a hidden TCR state
Walz’s group is known for building custom membrane environments that imitate the native surroundings of specific membrane proteins. “We can change the biochemical composition, the thickness of the membrane, the tension and curvature, the size—all kinds of parameters that we know have an influence on the embedded protein,” Walz says.
For this study, the researchers aimed to recreate a native-like environment for the TCR and watch how it behaved. They placed the receptor into a nanodisc, a small disc-shaped patch of membrane held in solution by a scaffold protein that wraps around the edge of the disc. The process was difficult, and “getting all eight of these proteins properly assembled into the nanodisc was challenging,” Notti says.
Earlier structural studies of the TCR had been done in detergent, which often strips away the membrane from the protein. Walz notes that this was the first study that returned the complex to a membrane environment.
Cryo-EM shows the receptor “spring open”
With the TCR in place, the team performed cryo-EM imaging. The images showed that, when resting, the T cell receptor takes on a closed, compacted shape. After an antigen-presenting molecule activates it, the receptor opens and extends outward, as if throwing its arms wide.
That result was unexpected. “The data that were available when we began this research depicted this complex as being open and extended in its dormant state,” Notti explains. “As far as anyone knew, the T cell receptor didn’t undergo any conformational changes when binding to these antigens. But we found that it does, springing open like a sort of jack-in-the-box.”
The researchers say two choices were crucial to seeing this behavior. First, they prepared the right membrane lipid cocktail to match the TCR’s in vivo environment. Second, they put the receptor back into that membrane using nanodiscs before cryo-EM imaging. They found the intact membrane acts like a stabilizing constraint that keeps the TCR in place until activation. When detergent removes the membrane, earlier studies may have accidentally released the latch on the jack-in-the-box and caused it to open too soon.
“It was important that we used a lipid mixture that resembled that of the native T cell membrane,” says Walz. “If we had just used a model lipid, we wouldn’t have seen this closed dormant state either.”
What this could mean for immunotherapies and vaccines
The team believes the new structural insight could help improve treatments that depend on T cell receptors. “Re-engineering the next generation of immunotherapies tops the charts in terms of unmet clinical needs,” Notti says. “For example, adoptive T cell therapies are being used successfully to treat certain very rare sarcomas, so one could imagine using our insights to re-engineer the sensitivity of those receptors by tuning their activation threshold.”
Walz says the work may also support vaccine development. “This information may be used for vaccine design as well,” Walz adds. “People in the field can now use our structures to see refined details about the interactions between different antigens presented by HLA and T cell receptors. Those different modes of interaction might have some implication for how the receptor functions—and ways to optimize it.”
Reference: 16 December 2025, Nature Communications.
DOI: 10.1038/s41467-025-66939-7
Never miss a breakthrough: Join the SciTechDaily newsletter.
Follow us on Google and Google News.