This post was originally published on here
A new method for engineering natural killer cells could make cancer immunotherapy more efficient, scalable, and affordable, potentially reshaping how these treatments are produced.
Chinese scientists have reported a new technique that makes it easier to genetically modify natural killer (NK) cells for use in cancer immunotherapy.
In the immune system, NK cells provide rapid, early protection against viruses and cancer, along with other important functions. That combination has made them a strong candidate for immunotherapy. One example is chimeric antigen receptor (CAR)-NK therapy, where researchers equip an NK cell with a lab-built receptor (a CAR) so it can spot a specific antigen on a cancer cell and then attack.
Many current CAR-NK approaches depend on mature NK cells taken from human sources such as peripheral blood or cord blood. This strategy can be difficult to scale because the cells vary widely from donor to donor, are harder to engineer efficiently, require costly handling, and often involve lengthy processing.
A New Strategy Using Stem and Progenitor Cells
A team led by Prof. Jinyong Wang from the Institute of Zoology of the Chinese Academy of Sciences has now introduced a method for producing induced (that is, lab-generated) NK (iNK) cells and CAR-engineered iNK (CAR-iNK) cells using CD34+ hematopoietic stem and progenitor cells (HSPCs) collected from cord blood.
The study was recently published in Nature Biomedical Engineering.
Past attempts to generate NK cells from cord blood-derived CD34+ HSPCs have been hindered by poor induction efficiency and the resulting cells’ functional immaturity. To overcome these barriers, the researchers shifted the genetic engineering to the earlier CD34+ HSPC stage, combining CAR transduction, efficient progenitor expansion, and NK-lineage commitment.
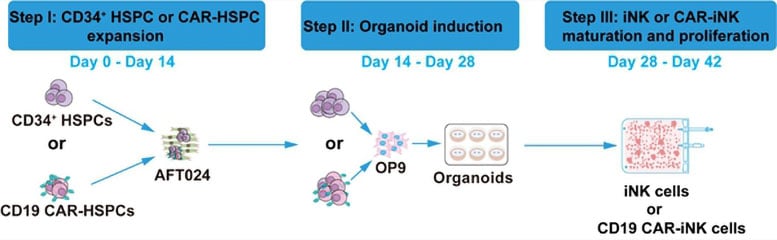
A Three-Step Engineering Process
The three-step process began with expanding CD34+ HSPCs (or CD19 CAR-transduced HSPCs) using irradiated AFT024 feeder cells, achieving approximately 800- to 1,000-fold growth within 14 days. Next, the expanded cells were co-cultured with OP9 feeder cells to form artificial hematopoietic organoid aggregates—structures that promote efficient NK lineage commitment and development. Finally, the cells committed to the NK lineage underwent maturation and proliferation, yielding highly pure iNK or CAR-iNK cells that expressed endogenous CD16.
Notably, the method demonstrated that a single CD34+ HSPC could produce up to 14 million iNK cells or 7.6 million CAR-iNK cells. In theory, just one-fifth of a standard cord blood unit could generate enough cells to supply thousands—or even tens of thousands—of therapeutic doses.
A major advance is the drastic reduction in the amount of viral vector required for CAR engineering. Compared with the viral load typically needed to engineer mature NK cells, the new method used only ~1/140,000 (by Day 42 of culture) to ~1/600,000 (by Day 49) as much vector.
Functional Validation in Cancer Models
In functional tests, the team confirmed that both iNK and CAR-iNK cells exhibited strong tumor-killing activity. In cell line-derived xenograft (CDX) and patient-derived xenograft (PDX) mouse models of human B-cell acute lymphoblastic leukemia (B-ALL), CD19 CAR-iNK cells suppressed tumor growth and prolonged the survival of the animals.
The new method not only boosted the induction efficiency of iNK and CAR-iNK cells but also substantially lowered the cost of CAR engineering, the researchers noted.
Reference: “Large-scale generation of iNK and CAR-iNK cells from CD34+ haematopoietic stem and progenitor cells for adoptive immunotherapy” by Fangxiao Hu, Jianhuan Li, Yao Wang, Yunqing Lin, Jingliao Zhang, Jiacheng Xu, Xiujuan Zheng, Qitong Weng, Xiaofei Liu, Yang Geng, Hongling Wu, Lijuan Liu, Huan Peng, Bingyan Wu, Dehao Huang, Chengxiang Xia, Tongjie Wang, Xin Du, Hui Zeng, Fang Dong, Yingchi Zhang, Xiaofan Zhu, Mengyun Zhang and Jinyong Wang, 7 October 2025, Nature Biomedical Engineering.
DOI: 10.1038/s41551-025-01522-5
Funding for the research was provided by the Ministry of Science and Technology of the People’s Republic of China and the National Natural Science Foundation of China, among others.
Never miss a breakthrough: Join the SciTechDaily newsletter.
Follow us on Google and Google News.