This post was originally published on here
Mars has an active, electrically charged surface where dust storms and spinning dust devils regularly move and reshape the landscape.
Mars is often portrayed as a dry, empty world, but the planet is far more active than it appears. Its thin atmosphere and dust-covered surface create conditions where electrical energy can easily build up. As dust storms and dust devils sweep across the landscape, they continuously alter the surface and drive energetic processes that have become a growing focus of scientific research.
Planetary scientist Alian Wang has been examining these electrically charged dust phenomena in a series of studies. Her most recent paper, published in Earth and Planetary Science Letters, investigates how these processes affect the isotopic composition of chemical elements on Mars, offering new insight into the planet’s geochemical behavior.
When dust storms surge across Mars, countless dust grains collide and rub against one another. This friction generates electrical charges that can accumulate to the point of producing electrostatic discharges (ESDs), capable of disrupting the planet’s tenuous atmosphere. Because Mars has much lower atmospheric pressure than Earth, these discharges occur more readily and may appear as faint, ghostly glows similar to auroras. In the process, they trigger a range of electrochemical reactions that reshape the planet’s chemical environment.
Simulating Mars in the Laboratory
Wang, a research professor of Earth, environmental, and planetary sciences at Washington University in St. Louis and a fellow of the university’s McDonnell Center for the Space Sciences, studies these dust-driven electrical processes to understand how they generate oxidized chemical compounds on Mars.
With support from NASA’s Solar System Working Program, her team developed two specialized planetary simulation chambers, PEACh (Planetary Environment and Analysis Chamber) and SCHILGAR (Simulation Chamber with InLine Gas AnalyzeR). These facilities allowed the researchers to reproduce Martian conditions and identify a wide range of chemical products, including volatile chlorine species, activated oxides, airborne carbonates, and (per)chlorates. Together, these substances play an important role in shaping Mars’ evolving geochemical system.
Dust, Electricity, and the Martian Chlorine Cycle
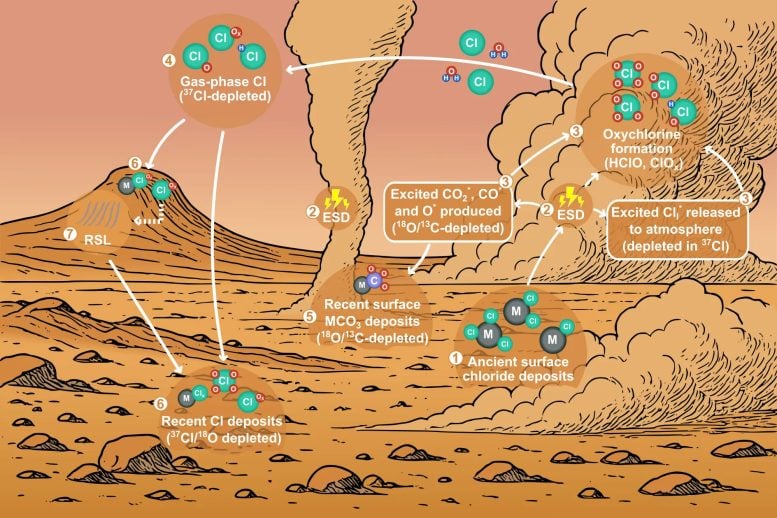
In earlier research, Wang and her colleagues identified dust driven electrical discharges as a key factor influencing the chlorine cycle on Mars. Large areas of the Martian surface contain chloride deposits, which are thought to be remnants left behind by ancient salty water.
By using a Mars simulation chamber equipped with multiple collection traps to establish mass balance, the researchers were able to measure and quantify the chemical products produced during these experiments. Their analysis showed that dust activity under the hot and dry conditions of Mars’ Amazonian period could form carbonates, (per)chlorates, and volatile chlorine compounds. These laboratory results closely align with chemical signatures detected by modern Mars orbiters, rovers, and lander missions.
Decoding the Isotopic Signatures of Mars Dust Activities
Wang’s team, comprising members from six universities in the United States, China, and the United Kingdom, analyzed the isotopic compositions of chlorine, oxygen, and carbon in ESD products. They found substantial and coherent depletion of heavy isotopes.
“Because isotopes are minor constituents in materials, the isotopic ratios can only be affected by the MAJOR process in a system. Therefore, the substantial heavy isotope depletion of three mobile elements is a ‘smoking-gun’ that nails down the importance of dust-induced electrochemistry in shaping the contemporary Mars surface-atmosphere system,” says Wang.
Each isotopic measurement, along with the previous quantifications, acts as a piece of a larger puzzle. This comprehensive view suggests that electrochemistry induced by Martian dust activities has sculpted the planet’s chemical landscape. These findings reinforce the hypothesis that Martian dust activities have played a crucial role in shaping the contemporary geochemistry of both the surface and the atmosphere.
A Global Model of Martian Chemistry
A conceptual model of Mars’ contemporary global chlorine cycle and airborne carbonate minerals emerges from this isotopic study. This model reveals a fascinating interplay between the electrochemical processes and secondary minerals on Mars’ surface and in its atmosphere.
It demonstrates how the heavy isotope depletions in three mobile elements are transferred from the dust-driven ESD products to the atmosphere and then re-deposited onto the surface, even percolating into the subsurface to form the next generation of surface minerals. The ongoing dust-driven electrochemistry throughout the Amazonian period has contributed to the progressive depletion of 37Cl, leading toward the very negative δ37Cl value (-51‰) observed by NASA’s Curiosity rover.
“Alian’s work is very important. This is the first experimental study to look at how electrostatic discharges can affect isotopes in a Martian environment. Isotopic signatures are like fingerprints, and they can be used to trace the processes that have influenced the chlorine cycle on Mars, which makes this study especially valuable,” notes Kun Wang, an associate professor of Earth, environmental, and planetary sciences at Washington University.
“While the experiments did not produce the extremely light Cl isotopic signatures measured by Mars rovers, they clearly show that electrostatic discharges can drive Cl isotopic fractionation in the right direction. This work is therefore an important step toward understanding the origin of these unusually light Cl signatures and the formation of perchlorate minerals on the Martian surface. It also highlights just how different Mars is from Earth, with very different atmospheric and surface processes controlling chemical reactions.”
Expanding Horizons in Planetary Science
Wang’s latest study coincides with new findings from NASA’s Perseverance rover that recorded 55 electric discharges on Mars during two dust devils and the convective front of two dust storms, published in Nature, in which her previous studies were cited as the chemical consequences of electrical discharges, affirming her role as a leading expert in understanding Mars’ electrified environment.
Her discoveries about the identification, quantification, and isotopic signature of (per)chlorates, amorphous salts, airborne carbonates, and volatile chlorine species all align with observations made from Mars missions, providing compelling evidence of dust-induced electrochemistry on Amazonian Mars.
Beyond Mars: Implications for Other Worlds
Wang’s research opens doors to new possibilities beyond Mars. Similar electrochemical phenomena might exist on other planets and moons such as Venus, the Moon, and the outer planetary systems. This expands the significance of her work, suggesting that electrochemistry induced by Martian dust, Venusian lightning, and energetic electrons on the Moon and outer planets are essential factors in planetary processes throughout the solar system.
“This research sheds light on an important facet of modern Mars: the interaction of the atmosphere and the surface. But it also tells us about how the chemistry of the surface has, in part, come to be—with valuable lessons for other worlds where triboelectric charging may take place, including Venus and Titan,” shares Paul Byrne, an associate professor of Earth, environmental, and planetary sciences at Washington University.
This innovative research direction electrifies our understanding of Mars, uncovering the potent role of dust activities in shaping its chemical landscape. Wang’s contributions propel planetary science forward, offering deeper insights into the dynamic forces at play on Mars and beyond. As we continue to explore, her discoveries provide the foundation for a richer understanding of our celestial neighbors, sparking curiosity and inspiring future missions to uncover the secrets held by other worlds in our solar system.
As Mars continues to reveal its secrets, groundbreaking research brings us closer to understanding our planetary neighbor, its history, and its potential to support life. The mysteries of Mars remind us that the Red Planet still holds many wonders, waiting to be fully explored.
Reference: “Isotope effects (Cl, O, C) of heterogeneous electrochemistry induced by Martian dust activities” by Neil C. Sturchio, Hao Yan, Alian Wang, W. Andrew Jackson, Huiming Bao, Chuck Y.C. Yan, Linnea J. Heraty, Yu Wei, Quincy H.K. Qu and Kevin S. Olsen, 18 December 2025, Earth and Planetary Science Letters.
DOI: 10.1016/j.epsl.2025.119784
Never miss a breakthrough: Join the SciTechDaily newsletter.
Follow us on Google and Google News.