Researchers have identified that G-quadruplexes promote harmful protein aggregation in neurodegenerative diseases. Blocking G4s with 5-ALA stopped Parkinson’s-like symptoms in mice, suggesting a promising path for early disease intervention.
Researchers at Kumamoto University have discovered a groundbreaking mechanism behind the formation of harmful protein aggregates that contribute to neurodegenerative diseases like Parkinson’s Disease.
The team, led by Professor Norifumi Shioda and Associate Professor Yasushi Yabuki, identified for the first time that unique RNA structures called G-quadruplexes (G4s) play a central role in promoting the aggregation of α-synuclein, a protein associated with neurodegeneration. By demonstrating that inhibiting G4 assembly could potentially prevent the onset of synucleinopathies, this discovery positions G4 as a promising target for early intervention in these diseases.
In a healthy state, α-synuclein typically regulates neuronal function. However, in neurodegenerative diseases, it aggregates together, leading to cell damage and motor symptoms.
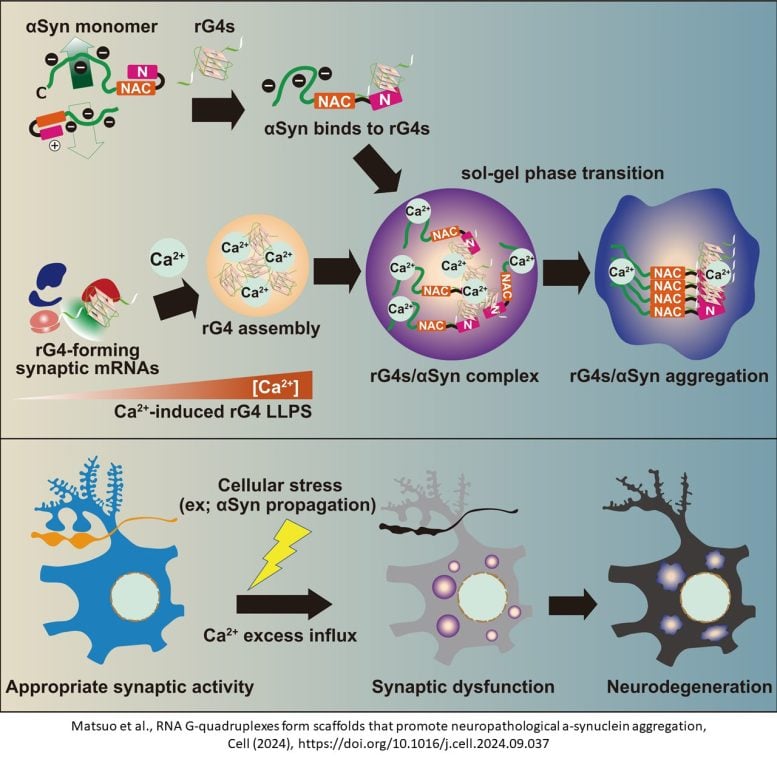
The researchers identified that G4s, four-stranded RNA structures that form in response to cellular stress, function as a “scaffold” that facilitates α-synuclein aggregation. Elevated calcium levels, often seen under stress, trigger G4 assembly, which then attracts α-synuclein, converting it into a harmful, aggregate-prone state.
A Novel Intervention: 5-ALA Treatment
The team went a step further, demonstrating a new approach to prevent this process. They administered 5-aminolevulinic acid (5-ALA), a compound that blocks G4 formation, to model mice exhibiting Parkinson’s-like symptoms. Impressively, 5-ALA treatment not only prevented α-synuclein aggregation but also halted the progression of motor symptoms, a promising sign for potential therapies targeting early-stage neurodegeneration.
This breakthrough could significantly advance treatments aimed at neurodegenerative diseases by focusing on G4 regulation. Since G4s are also implicated in other diseases such as Alzheimer’s Disease, this discovery may broaden the impact of such treatments beyond Parkinson’s Disease. These findings also shed new light on preemptive strategies to combat neurodegeneration and improve the quality of life for aging populations.
Reference: “RNA G-quadruplexes form scaffolds that promote neuropathological α-synuclein aggregation” by Kazuya Matsuo, Sefan Asamitsu, Kohei Maeda, Hiroyoshi Suzuki, Kosuke Kawakubo, Ginji Komiya, Kenta Kudo, Yusuke Sakai, Karin Hori, Susumu Ikenoshita, Shingo Usuki, Shiori Funahashi, Hideki Oizumi, Atsushi Takeda, Yasushi Kawata, Tomohiro Mizobata, Norifumi Shioda and Yasushi Yabuki, 18 October 2024, Cell.
DOI: 10.1016/j.cell.2024.09.037
Funding: Japan Agency for Medical Research and Development, Japan Society for the Promotion of Science, Fusion Oriented REsearch for disruptive Science and Technology, MEXT Promotion of Development of a Joint Usage/Research System Project: Coalition of Universities for Research Excellence Program (CURE), Pharmacological Research Foundation, Tokyo, Kowa Life Science Foundation, Narishige Neuroscience Research Foundation, Mochida Memorial Foundation for Medical and Pharmaceutical Research, Astellas Foundation for Research on Metabolic Disorders, the Foundation of SBI Pharmaceuticals Co., Ltd., Takeda Science Foundation
This post was originally published on here







