Whole genome sequencing is creating bespoke cancer cures.
Yuichiro Chino/Getty
Like many cancer patients, Michael Wolff wanted answers. But, like many cancer patients in 2015, he wasn’t getting them.
After years of lymphoma treatment, the renowned jazz musician was still sick and unable to play. His wife insisted that he seek a second opinion at Memorial Sloan Kettering Cancer Center in New York City.
MSK doctors found that, rather than lymphoma, Wolff had histiocytic sarcoma: a rare blood cancer affecting 300 Americans each year. He was referred to Dr. Mrinal Gounder, a sarcoma medical oncologist and early drug development specialist. The pair—now friends—recounted their first meeting for Newsweek.
“Look,” Wolff recalls telling Gounder, “if you don’t know about this, get me to the doctor seeing the most of these.”
“I’ve seen the most of these,” Gounder replied.
“How many?”
“Ten.”
Wolff didn’t ask what happened to those 10 patients. It became clear just how little doctors—even experts—knew about his rare cancer. But Gounder had an idea: Perhaps Wolff’s genes could point them in the right direction.

Dr. Mrinal Gounder, a sarcoma medical oncologist at Memorial Sloan Kettering Cancer Center (MSK) in New York City, with Michael Wolff, a renowned jazz musician and a cancer survivor
Elliott Atkinson
Genetic sequencing was new at the time, akin to “science fiction work,” Gounder said. The test revealed Wolff had a mutation that had been recently linked to a common lung cancer. Gounder believed a pill called Mekinist, approved to treat certain melanomas, could be effective.
Wolff was a bit skeptical—after all that chemo, could a pill really be the answer?
“What’s the research on this medicine?” Wolff asked.
Gounder answered: “You are the research.” No one with his specific cancer had taken Mekinist before. But within two days of taking the pill, all of Wolff’s symptoms had vanished. Within 10 days, a PET scan showed an 80 percent reduction in his Stage four tumors.
When Gounder first met Wolff, he estimated his new patient had two months to live. Now, nearly a decade later, Wolff’s cancer has not returned.
Wolff’s case was published in the New England Journal of Medicine as an early example of precision cancer treatment. Nearly 10 years later, researchers can sequence a person’s entire genome in mere days. The science has progressed further than anyone believed possible and, depending on who you ask, further than necessary.
Whole genome sequencing, which is also being used widely to improve treatments of difficult-to-diagnose diseases as well as for genetic screening of newborns, has given hope to patients with certain rare cancers. Leaders in the space believe it could eventually shape health care for everyone. But access remains limited due to high costs and regulatory hurdles, while some professionals question the purpose of such extensive testing. After all, we can only interpret about 2 percent of the data that WGS returns.
Exploring the remaining 98 percent—dubbed the “dark genome”—might lead to cures for historically incurable ailments, experts told Newsweek. It will also require tremendous resources.

At MSK’s Integrated Genomics Lab, robots convert RNA and DNA into a readable form for sequencing machines.
Renae Whissel
What Is Genetic Sequencing?
Cancer is a disease of the genome, according to Dr. Elli Papaemmanuil, computational oncologist at MSK. By looking at the genome—all the genetic material in an organism—researchers can gather clues about what caused certain cancers, and even how they might respond to treatment.
Each of a human being’s cells contain a complete set of DNA, Papaemmanuil explained. That DNA contains instructions for the cell to keep the body working properly. Some mutations, such as BRCA genes which can make women susceptible to breast cancer, are inherited from a person’s parents. But DNA can also accumulate mutations over a lifetime: some are natural parts of aging, and others come when we “pressure test” the system, like by smoking.
Mutated DNA can give a cell faulty instructions, leading it to behave differently. Cancer occurs when these dysregulated cells proliferate and take over. Think of the genome like a cookbook, Papaemmanuil said. Each cell is preparing a recipe, and its DNA provides step-by-step directions. Mutations can be small, like a misspelled ingredient, or significant, like an entire page that was printed back to front.
With modern genomic sequencing technology, it is possible to identify the exact “letter” or “page” that altered the cells—and, in some cases, deploy a targeted therapy to correct them.
To understand cancer mutations, scientists needed a reference point or “code book of DNA,” Papaemmanuil said. Mapping the first human genome was no easy feat. The Human Genome Project took approximately 13 years, $3 billion and the combined brainpower of 20 universities and research centers across six countries. In 2003, the finished product laid the foundation for a new era of research for cancer and rare diseases.
More than two decades later, researchers have made significant progress with genomic sequencing. They’ve developed technology that can scan patients’ DNA at scale and have catalogued the most common cancer-causing mutations. This has allowed for more precise, personalized cancer treatment, like the kind Wolff received at MSK.
When MSK-IMPACT, the test that diagnosed Wolff, was launched in 2015, it was “a paradigm-shifting moment in cancer,” Papaemmanuil recalled.
“For many cancer types, outcomes have completely changed,” she said. “We have seen tumor types that were incurable now be cured without even giving them chemotherapy.”
MSK-IMPACT currently identifies more than 500 cancer mutations, and is one of multiple next-generation sequencing tests on the market. Before NGS, it was not feasible to develop drugs for rare genomic alterations because each mutation required a specific diagnostic test, according to Dr. David Solit, director of developmental therapeutics at MSK and codirector of its molecular oncology center. Choosing the right test for a mutation that only 1 in 1,000 patients had was like searching for a needle in a haystack.

Dr. David Solit, codirector of the molecular oncology center at MSK, taking Newsweek on a tour of his lab.
Renae Whissel
But with NGS tests, oncologists can check for hundreds of mutations at once. Patterns began emerging, so researchers could begin developing targeted drugs. There has been a surge in these therapeutics over the past five years, Solit told Newsweek. To date, more than 16,000 people have had their genomes sequenced with MSK-IMPACT. Families have also benefited from knowing their genetic histories—when hereditary mutations are found, they can intervene early.
“What was potentially science fiction 20 years ago became common practice for the major cancer hospitals,” Papaemmanuil said.
How Is Whole Genome Sequencing Unique?
But even with sophisticated science on our side, we’ve barely scratched the surface. Each human genome contains nearly 3 billion base pairs (the units that compose DNA). That amount of data is difficult to fathom, but the National Human Genome Research Institute puts it into perspective: One single human genome produces 200 gigabytes of data, equivalent to 200 DVDs of the 1975 film Jaws.
While getting a standard NGS test is like watching the most important scenes, whole genome sequencing is like watching the full movie, all 200 times. A standard NGS test might produce two or three relevant data points about a patient’s tumor, according to Papaemmanuil; a WGS test could produce 15,000.
“That really speaks to what we leave behind,” Papaemmanuil said. “Many patients are offered a genetic profiling test in the promise of precision medicine, but at the end of that, the report could be blank—not because their tumors don’t have mutations, but because our diagnostic tests are not designed to look at mutations their tumors have.”
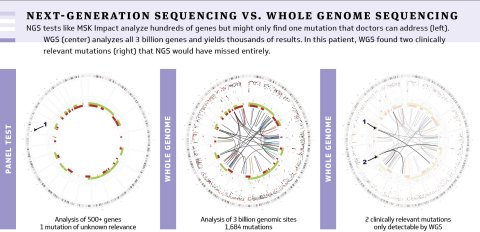
NGS tests like MSK Impact analyze hundreds of genes but might only find one mutation that doctors can address (left). WGS (center) analyzes all 3 billion genes and yields thousands of results. In this patient, WGS found two clinically relevant mutations (right) that NGS would have missed entirely.
Memorial Sloan Kettering Cancer Center
Sometimes, the whole genome reveals another treatment option for patients with rare or advanced metastatic diseases that aren’t responding to existing therapies. Most NGS tests were designed to look at the most common tumor types—breast, colorectal, prostate, lung—but they still exclude between a third and a fourth of cancer patients, Papaemmanuil continued.
Why, then, didn’t scientists skip straight to WGS initially? Preliminary studies showed the most obvious mutational targets clustered in about one-tenth of a percent of the genome, according to Dr. Marcin Imieliński, director of the Cancer Genomics Research Program at NYU Langone Health’s Perlmutter Cancer Center, and a core faculty member at the New York Genome Center. The early tests chose to zoom in on that significant sliver.
Plus, sequencing and storage costs were too prohibitive to consider WGS in the early days of genetic test development, according to Dr. Michael Berger, MSK’s chief of pathology and laboratory medicine, who also codirects the molecular oncology center with Solit. Standard NGS points to a standard precision oncology treatment option for about one-third of patients who are sequenced, according to Solit. That still leaves two-thirds who don’t benefit, either because there is no targeted drug for their mutation, or because the assays weren’t broad enough to detect them.

Dr. Michael Berger, codirector of the molecular oncology center at MSK, during a tour of his lab.
Renae Whissel
WGS could “do better” for the two-thirds, Solit said. Indeed, Papaemmanuil said her team finds mutations in 100 percent of cancer patients—about half of which are clinically relevant biomarkers that can inform treatment.
Dr. Wesley Walker, director of genomics and personalized health at AdventHealth’s Central Florida Division, has also seen those benefits firsthand.
“When you put whole genome into the mix, it actually changes the therapy a third of the time,” Walker told Newsweek. “That’s significant.”
In oncology, Walker has found WGS to be particularly promising in leukemias, sarcomas, central nervous system cancers and pediatric cancers. Dr. Andrew Kung, chair of the pediatrics department at MSK, also spoke of WGS’ potential in childhood cancers. Unlike adults, children’s genetic mutations are rarely caused by lifelong exposures to carcinogens. Tests designed to detect common lung cancer mutations, for instance, are rarely useful in kids. However, WGS provides a deeper dive and has become the standard workup for children treated at MSK. The health system has sequenced whole genomes for nearly 1,000 pediatric patients and can return results in under a week.
WGS can point to new approaches when first-line treatments have failed. Standard tests found “nothing actionable” in a teen with a rare cancer, but when MSK sequenced his whole genome, it was “littered with mutations,” Kung said.
Such high mutation loads are rare in pediatric cancers, but WGS has identified them in certain individuals, and it has led doctors to unconventional solutions, according to Kung. This teen was put on immune checkpoint inhibitors. After three doses, his tumor began to shrink. Once it was gone, it never came back.
“Only through the benefit of whole genome sequencing were we able to find a curative therapy that no other technology would have pointed us to,” Kung said.

Dr. Andrew Kung (shown above with Newsweek Health Care Editor Alexis Kayser) says most oncologists believe WGS will be standard cancer treatment 10 years from now, though cost must come down further to make that possible.
Elliott Atkinson
Dr. Jeffrey Klco has had similar findings at St. Jude Children’s, the director of the hematopathology and molecular pathology division told Newsweek. “Without exaggeration, almost every week we find or report something that we didn’t really know about, some new alteration,” Klco said. “Sometimes they spur research opportunities.”
St. Jude began its genomics work about a decade ago and now provides WGS to between 400 and 500 patients per year, said Klco. The Memphis, Tennessee-based research hospital aims to sequence every patient with a tumor, and it’s the only organization in the country that covers the cost.
Forbes recently named St. Jude the top medical charity in America, amassing $2.57 billion in private donations in the most recent fiscal year. But few hospitals have the funds to fuel a WGS lab—much less to pay for each patient’s test.
“If you ask most oncologists, ‘What is the first go-to test that we’ll do 10 years from now?’ I think the answer from most people would be WGS,” Kung said. “Several things have to happen before that actually becomes reality.”
What Are the Challenges Facing Whole Genome Sequencing?
The price of WGS has dropped significantly since that first $3 billion genome was mapped. Now, most experts who spoke with Newsweek placed the total cost of a test between $7,000 and $10,000. This is on par with most cancer tests, though not affordable enough to become standard, according to Kung.
“Cost is a direct relationship to technology advances,” Dr. Steven Barnard—chief technology officer of Illumina, the market leader in DNA sequencing machines—told Newsweek.
The cost of the technology has become much less prohibitive over the past two decades, Barnard said. In 2002, it still cost approximately $100 million to sequence one human genome, according to the NHGRI. In 2022, it cost under $1,000. Certain Illumina machines—those with the greatest volume—can run samples for a few hundred dollars each.

An Illumina NovaSeq X Plus sequencer completes its run at an MSK lab. This machine can sequence 25 to 50 billion molecules in a single run, which takes two days, according to Dr. Neeman Mohibullah, director of the Integrated Genomics Operation at MSK.
Renae Whissel
Genome sequencing has grown so rapidly that this year, it will require approximately 40 exabytes of data storage, according to the NHGRI. For reference, every word ever spoken by human beings could be stored in 5 exabytes. It is expensive to store and interpret that amount of data, and critics of WGS argue that much of it is unnecessary.
“There are people who are worried that we are going to have too much data to interpret these genomes and to turn them around in time to give oncologists the answers that they really want and need—but also maybe [identify] other things that they weren’t even expecting,” Imieliński said.
Dr. Brenda Wilson is one of those people. She’s a medical doctor, public health researcher and associate dean of the medical school at Memorial University in Newfoundland and Labrador, Canada—and has voiced concerns about NGS, including WGS, since 2017.
Clinical utility is necessary when providing such extensive, life-altering information, Wilson told Newsweek. While she believes the information can be useful in some cases—helping people plan ahead and “come to settled decisions about their lives,” for example —she is concerned that a patient seeking answers in one area may, in turn, get a laundry list of new things to worry about. The science is new enough that many answers only raise more questions.
“You shouldn’t offer people information without genuine ability to do something with it,” Wilson said.
Papaemmanuil took a different approach, saying: “If I were a cancer patient, I’d feel more comfortable if a doctor told me, ‘I looked at everything and I didn’t find anything that I can do right now,’ than, ‘I looked at 1 percent of your genome, and I think there’s nothing for you.”
Still, there are barriers to scaling the technology—especially in the United States. Molecular testing is highly regulated by the FDA, and its current stipulations aren’t exactly conducive to WGS, experts told Newsweek. Originally, geneticists used to look at one mutation at a time, develop separate tests for each, and then the individual tests then got approved by the FDA.
“That’s not a great fit, especially as we move into the era of [WGS],” Imieliński said. “It becomes a little bit impractical to be regulating every single analyte that we’re measuring as a separate device.”

Dr. Marcin Imieliński at work in his lab.
Renae Whissel
Currently, many health systems operate their WGS programs without FDA approval. Patients enroll in clinical trials to partake. It’s challenging to meet regulatory criteria when the subject matter is unprecedentedly vast, according to Kung.
“When you can see everything across the entire genome, how do you demonstrate rigor in terms of every single element that you want to test for?” Kung said. “The path to approval for looking across the entire genome is uncharted territory.”
The FDA told Newsweek by email that the risks associated with these modern laboratory developed tests, or LDTs, are much greater than LDTs used decades ago. Business practices have changed; large laboratories now accept samples that have been shipped from across the country, relying on high-tech instruments and using their findings “to help guide critical health care decisions.”
While these tests were once used in local, specialized populations, they are now being used for larger, more diverse populations, according to the FDA. The agency is aware of “numerous” examples of “potentially inaccurate, unsafe, ineffective or poor quality” tests, including some used to manage rare diseases, identify cancer risk and select cancer treatment, per an April news release explaining the FDA’s decision to heighten oversight. “The agency cannot stand by while Americans continue to rely on results of these tests without assurance that they work,” FDA Commissioner Dr. Robert Califf said in April.

Dr. Neeman Mohibullah, director of the Integrated Genomics Operation at MSK, gives Newsweek a tour of her lab, which processes between 30,000 and 40,000 samples per year.
Renae Whissel
The issue trickles into insurance reimbursement. Currently, only one WGS assay has been approved by Medicare: ChromoSeq, a hematologic test developed by Dr. Eric Duncavage, division chief of genomic and molecular pathology, and his team at Washington University School of Medicine in St. Louis, where the first cancer genome was sequenced. To get approval, the team proved that WGS yielded roughly 20 percent more actionable information than conventional testing methods. But it’s not always easy to provide such proof for extremely rare cancers, according to Papaemmanuil.
“Payers are usually looking for clinical utility, and it’s very easy to design a clinical utility study for breast cancer—you have the population size, you have the treatments, you have statistical power,” she said. “When you’re looking at rare cancers, the notion of clinical utility is very different.”
What Is the Potential of Whole Genome Sequencing?
Multiple countries are forming nationally funded whole genome initiatives: the United Kingdom, Denmark, Germany, France and Sweden are among them. “In Sweden, we are lucky,” Dr. Richard Rosenquist Brandell, director of Genomic Medicine Sweden, told Newsweek. “We have a national infrastructure for life sciences that is an investment into high throughput technologies, everything from genomics, proteomics, imaging, you name it.”
Despite being a small country with 10 million inhabitants and seven university hospitals, Sweden has completed 8,000 whole genomes for rare disease and approximately 20,000 cancer sequencing panels, according to Brandell. WGS is cheaper there—averaging less than $4,000—and all tests are reimbursed by the Swedish health care system.
Each research center is connected to a national genomic platform where data is stored and shared. This will allow them to learn from one another and collaborate on future treatments, Brandell said, noting that this is “the golden era” for geneticists.

Researchers from the Auragen laboratory prepare the sequencing of human genomes to better identify rare diseases, in Lyon, central-estern France, on February 23, 2022. – Nearly 8,000 rare diseases have been identified to date and they affect about three million people in France, the vast majority of whom are children.
JEAN-PHILIPPE KSIAZEK/AFP/Getty
In the United States, our health care system is composed of independent entities. But there’s still a sense of community among research centers. For example, investigators from other centers can apply for access to the cloud where St. Jude stores its data. MSK hopes to create the world’s largest WGS data set of pediatric cancers and make it publicly available, according to Papaemmanuil . Washington University is working with other academic labs to help them set up assays of their own, Duncavage said.
But the cost will remain a prohibitive factor for smaller hospitals. The Illumina sequencer is still expensive enough that Washington University schedules service like an airline, according to Duncavage. It is only cost effective when every “seat” in the sequencer is filled.
“If we run bigger batches, the cost per case is lower, but it also means the turnaround time is slower, and so we’re constantly trying to optimize that balance between turnaround time and cost per sample,” Duncavage said. “We have a pretty big institution here, we see a lot, so we can run the sequencing two or three times a week at a reasonable cost. But [at] a smaller center, it’d be challenging to have enough volume to fill the sequencer to capacity.”
What’s Next for Genetic Sequencing and Health Care?
Down the road, Walker believes WGS could inform a person’s health and longevity—regardless of whether they have an active cancer. Early trials have sequenced the whole genomes of newborns. “We think that a person’s whole genome is going to shape their care over the course of their life,” Walker said.
NGS has certainly changed the course of life for Wolff, who has now lived nearly a decade cancer-free and is back to playing jazz. He recently opened for Billie Eilish at Madison Square Garden with his sons, Nat and Alex. But “no matter what I’ve done in my career,” Wolff said, “being part of this experiment, having big success and changing so many other lives means the most to me.”
As for Gounder—Wolff’s physician who now attends his gigs—he’s peppering his curiosity with a healthy dose of doubt. “Cancer biology is extraordinarily complicated, and I think we are just learning to scratch the surface,” Gounder said. “Whether it’s a common cancer or it’s an ultra-rare cancer, you have to approach all of this with a sense of humility.”
“At the end of the day, life and biology are far smarter than all of us.”

ON TARGET: Genetic sequencing is creating bespoke cancer cures
Photo-illustration by Andriy Onufriyenko/Getty
fairness meter
This post was originally published on here







