This post was originally published on here
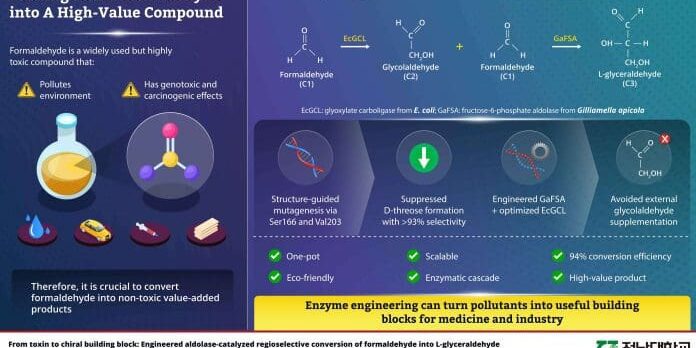
Researchers at Chonnam National University have developed an engineered enzyme system that converts highly toxic formaldehyde into L-glyceraldehyde with 94 percent efficiency in a sustainable water based process. The breakthrough published in November 2025 demonstrates how enzyme engineering can transform dangerous industrial pollutants into valuable pharmaceutical compounds through selective conversion.
Dr. Taner Duysak and Professor Jeong-Sun Kim led the research team at the Department of Chemistry and the Host-Directed Antiviral Research Center. Their work involved structurally engineering fructose-6-phosphate aldolase derived from Gilliamella apicola bacteria. The enzyme catalyzes carbon to carbon bond formation through an aldol condensation reaction between glycolaldehyde and formaldehyde. Initial approaches produced significant amounts of D-threose as a byproduct, but structure guided mutagenesis through Ser166 and Val203 mutations lowered D-threose formation with over 93 percent selectivity under mild aqueous conditions.
The research team achieved in situ glycolaldehyde production from formaldehyde by coupling engineered GaFSA to an optimized glyoxylate carboligase from E. coli. This one pot eco friendly and scalable enzymatic cascade reached approximately 94 percent conversion efficiency from 25 millimolar formaldehyde at pH 7.5 and 40 degrees Celsius with minimal byproducts. According to Dr. Duysak, the entire reaction proceeds in water under ambient pressure without toxic reagents or organic solvents, requiring only natural cofactors for EcGCL activity.
Formaldehyde remains a common chemical used across various industries as a disinfectant, resin precursor and synthetic intermediate. The volatile compound poses serious health and environmental threats due to its genotoxic and carcinogenic properties. Industrial facilities release formaldehyde emissions into air and water systems, while the compound also forms during natural metabolic processes including enzymatic demethylation of nucleic acids and proteins. Exposure through inhalation or ingestion may result in convulsions and renal failure, whereas chronic exposure links to increased cancer risks including nasopharyngeal cancer and leukemia.
Traditional formaldehyde detoxification relies on glutathione dependent pathways where the compound reacts with glutathione to form S-hydroxymethylglutathione. Alcohol dehydrogenase then oxidizes this intermediate to S-formylglutathione, which undergoes further metabolism by S-formylglutathione hydrolase to produce formate. However, these natural detoxification mechanisms often struggle with high formaldehyde concentrations in industrial waste streams. Chemical conversion methods typically require harsh conditions, expensive catalysts or generate additional toxic byproducts.
The engineered enzyme approach offers multiple advantages over existing detoxification strategies. L-glyceraldehyde serves as a renewable raw material functioning as a crucial precursor for rare sugars such as L-sorbose and L-psicose. As a C3 compound, it plays key roles in many biochemical pathways. Pharmaceutical companies utilize chiral intermediates derived from L-glyceraldehyde in drug development processes. The compound facilitates development of novel therapeutics with antibiotic, anti-cancer and other medicinal effects.
Recent advances in synthetic biology have expanded possibilities for one carbon chemistry. Formaldehyde represents an emerging C1 resource that can be derived from carbon monoxide, carbon dioxide, formic acid, methane and methanol through biological or chemical means. Formaldehyde converting enzymes including formolase, benzaldehyde lyase and pyruvate aldolase function as key biocatalysts in synthesizing value added chemicals from C1 feedstocks. Despite considerable research progress, highly efficient catalyst systems enabling large scale industrial applications remain limited.
Several research groups worldwide have pursued similar formaldehyde conversion technologies. Scientists at the Shenzhen Institute of Synthetic Biology developed mass spectrometry based screening methods for glycolaldehyde synthase, an engineered enzyme from Pseudomonas putida that condenses formaldehyde into carbohydrate molecules. Their high throughput approach identified mutants with 3.7 fold increases in catalytic efficiency. Meanwhile, researchers at Technical University of Munich engineered formolase variants to improve formaldehyde tolerance in E. coli through directed evolution, achieving 30 percent decreases in substrate binding constants.
The Chonnam National University team emphasizes environmental detoxification applications alongside green chemistry benefits. Industrial facilities could implement the enzyme system to safely remove formaldehyde from waste streams before discharge. The water based process operates at ambient temperature and pressure without requiring specialized equipment or hazardous chemicals. Natural cofactors needed for enzyme activity can be regenerated through coupled enzyme systems, reducing operational costs. The technology aligns with circular economy principles by converting waste pollutants into commercially valuable products.
Pharmaceutical manufacturing stands to benefit significantly from L-glyceraldehyde production capabilities. Current chemical synthesis routes to chiral building blocks often require multiple steps, expensive catalysts and generate racemic mixtures requiring separation. Enzymatic production delivers enantiopure L-glyceraldehyde directly, eliminating costly purification steps. The compound serves as a starting material for synthesizing complex drug molecules, particularly those requiring specific three dimensional configurations for biological activity.
Rare sugar production represents another promising application. L-sorbose finds use as a low calorie sweetener and pharmaceutical intermediate, while L-psicose functions as a rare monosaccharide with potential health benefits. Both compounds command premium prices due to limited availability and difficult synthesis routes. Enzymatic conversion of formaldehyde waste into rare sugar precursors could establish economically viable production methods while simultaneously addressing pollution concerns.
The research builds on broader expertise in enzyme engineering at Chonnam National University. Previous work by the same research group demonstrated engineered pyruvate aldolase applications for converting methanol derived formaldehyde into 2-keto-4-hydroxybutyrate, an intermediate for amino acids and hydroxy carboxylic acids. That project received support from the C1 Gas Refinery Program funded by the Ministry of Science and ICT. The accumulated knowledge in structure based rational design and directed evolution informed the current breakthrough.
Looking ahead, the researchers envision industrial implementations within the next decade. Chemical manufacturers could integrate the enzyme system into existing formaldehyde handling facilities to capture emissions and convert them into marketable products. Environmental remediation companies might deploy the technology at contaminated sites where formaldehyde poses groundwater or soil pollution risks. Pharmaceutical contract manufacturers could adopt the process for producing chiral building blocks more sustainably than conventional synthetic methods.
Scaling challenges remain before widespread commercial adoption. Enzyme production costs must decrease through improved expression systems or alternative manufacturing approaches. Reaction engineering studies need to optimize bioreactor designs for maximum conversion rates and product concentrations. Downstream processing methods require development to efficiently recover and purify L-glyceraldehyde from reaction mixtures. Regulatory approval processes for enzyme based industrial applications may present additional hurdles depending on specific use cases.
The research appeared online on October 21, 2025 before formal publication in the International Journal of Biological Macromolecules on November 1, 2025. The paper demonstrates proof of concept for regioselective formaldehyde conversion using engineered aldolase catalysis. Future work will focus on further improving enzyme performance, exploring additional product applications and developing integrated bioprocessing systems. The team collaborates with industrial partners to evaluate commercial feasibility and identify priority application areas.
Chonnam National University, established in 1952 as Korea’s first national university, maintains strong research programs in biotechnology and sustainable chemistry. The institution contributed to national development through advances in enzyme technology, agricultural biotechnology and pharmaceutical sciences. Faculty members lead research groups investigating enzyme engineering, biocatalysis and green chemistry applications. Graduate programs train researchers in protein engineering, structural biology and industrial biotechnology.
Similar enzyme engineering approaches could address other toxic chemical pollutants requiring conversion or detoxification. Acrolein, another reactive aldehyde present in industrial emissions and combustion products, causes similar health concerns as formaldehyde. Engineered enzymes might convert acrolein into useful chemicals like glyceraldehyde or glyceric acid through aldol reactions. Acetaldehyde from ethanol fermentation could undergo enzymatic condensation to produce C4 compounds with industrial applications. The fundamental strategies developed for formaldehyde conversion may extend to broader classes of reactive carbonyl pollutants.
Environmental regulations increasingly push industries toward cleaner production methods and waste valorization strategies. Technologies converting toxic byproducts into valuable chemicals help companies meet sustainability goals while generating additional revenue streams. The enzyme engineering approach aligns with green chemistry principles emphasizing pollution prevention, atom economy and renewable feedstocks. Regulatory frameworks supporting circular economy initiatives could accelerate adoption of such technologies across multiple industrial sectors.