This post was originally published on here
Scientists have turned brain cells into tiny light sources, revealing the brain at work like never before.
About ten years ago, scientists began exploring an unconventional idea for studying the brain: using bioluminescent light to make neural activity visible. Instead of shining light onto the brain from the outside, they wondered if neurons could be engineered to glow on their own.
“We started thinking: ‘What if we could light up the brain from the inside?’” said Christopher Moore, a professor of brain science at Brown University. “Shining light on the brain is used to measure activity — usually through a process called fluorescence — or to drive activity in cells to test what role they play. But shooting lasers at the brain has down sides when it comes to experiments, often requiring fancy hardware and a lower rate of success. We figured we could use bioluminescence instead.”
Launching the Bioluminescence Hub
That idea helped lead to the creation of the Bioluminescence Hub at Brown’s Carney Institute for Brain Science, which officially launched in 2017. The effort was supported by a major grant from the National Science Foundation and built on collaborations among Moore (associate director of the Carney Institute), Diane Lipscombe (the institute’s director), Ute Hochgeschwender (at Central Michigan University), and Nathan Shaner (at the University of California, San Diego).
The group set out to design and share new neuroscience tools by enabling nervous system cells to generate light and respond to it.
[embedded content]
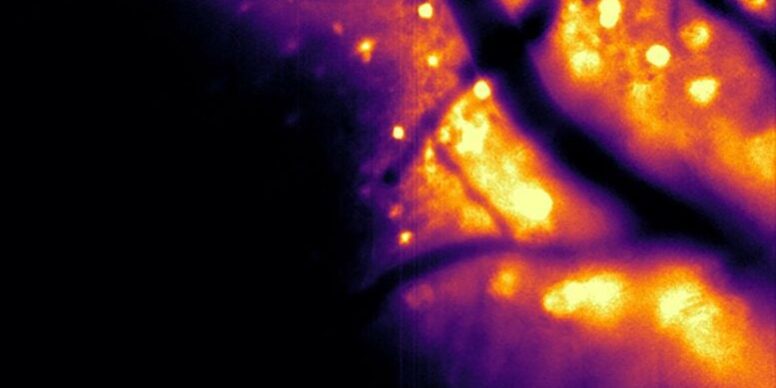
This video captures HeLa cells in the lab that have been engineered to self-create light.
Introducing CaBLAM, a New Bioluminescent Brain Tool
In a study published in Nature Methods, the researchers described a bioluminescent imaging system they recently created. Known as the Ca2+ BioLuminescence Activity Monitor (or “CaBLAM,” for short), the tool is capable of capturing activity at the level of individual cells and even smaller cellular structures. It performs well in mice and zebrafish, supports recordings that last for multiple hours, and eliminates the need for external illumination.
Moore said that Shaner, an associate professor of neuroscience and pharmacology at U.C. San Diego, led the development of the molecular device that made CaBLAM possible. “CaBLAM is a really amazing molecule that Nathan created,” Moore said. “It lives up to its name.”
Why Tracking Brain Cell Activity Matters
Understanding how living brain cells behave over time is essential for studying how biological systems function, Moore explained. Today, most researchers rely on imaging methods that use fluorescence-based genetically encoded calcium-ion indicators.
“In the way fluorescence works, you shine light beams at something, and you get a different wavelength of light beams back,” said Moore, who leads the Bioluminescence Hub. “You can make that process calcium-sensitive so you can get proteins that will shift back a different amount or different color of light, depending on whether or not calcium is present, with a bright signal.”
The Drawbacks of Fluorescent Brain Imaging
Although fluorescent probes are widely used, Moore said they come with serious limitations when applied to brain research. Prolonged exposure to intense external light can damage cells. High-powered illumination can also alter fluorescent molecules so they stop emitting sufficient light, a process known as photobleaching that restricts how long experiments can last. In addition, delivering light into the brain typically requires invasive equipment such as lasers and fiber optics.
Why Bioluminescence Works Better
Bioluminescent imaging avoids many of these problems. In this approach, light is produced internally when an enzyme breaks down a specific small molecule. Because no bright external light is required, there is no photobleaching and no phototoxic effect, making the method safer for brain tissue.
It also improves visibility.
“Brain tissue already glows faintly on its own when hit by external light, creating background noise,” Shaner said. “On top of that, brain tissue scatters light, blurring both the light going in and the signal coming back out. This makes images dimmer, fuzzier, and harder to see deep inside the brain. The brain does not naturally produce bioluminescence, so when engineered neurons glow on their own, they stand out against a dark background with almost no interference. And with bioluminescence, the brain cells act like their own headlights: You only have to watch the light coming out, which is much easier to see even when scattered through tissue.”
Moore noted that while researchers have discussed using bioluminescence to study brain activity for decades, previous attempts failed to generate light bright enough for detailed imaging. That limitation has now been overcome.
The Breakthrough Behind CaBLAM
“The current paper is exciting for a lot of reasons,” Moore said. “These new molecules have provided, for the first time, the ability to see single cells independently activated, almost as if you’re using a very special, sensitive movie camera to record brain activity while it’s happening.”
Using CaBLAM, scientists can monitor the behavior of individual neurons inside living laboratory animals, including activity within different parts of a single cell. In the study, the team presented results from a recording session that ran continuously for five hours, something that would not be possible with traditional fluorescence-based techniques.
“For studying complex behavior or learning, bioluminescence allows one to capture the entire process, with less hardware involved,” Moore said.
Expanding the Possibilities of Brain Research
The CaBLAM project is part of a larger effort at the Bioluminescence Hub to develop new ways to observe and influence brain activity. One ongoing project uses a living cell to emit a flash of light that can be detected by a nearby cell, allowing neurons to communicate using light itself (what Moore calls, “rewiring the brain with light”). The team is also working on methods that use calcium to control cellular behavior.
As these projects advanced, researchers realized that brighter and more effective calcium sensors were essential to all of them. Improving those sensors has since become a central focus, Moore said.
“We made sure that as a center that’s trying to push the field forward, we created the necessary component pieces,” Moore said.
Beyond the Brain
Moore believes CaBLAM could eventually be used to study activity in other parts of the body, not just the brain.
“This advance allows a whole new range of options for seeing how the brain and body work,” Moore said, “including tracking activity in multiple parts of the body at once.”
He added that the project highlights the strength of collaborative science. At least 34 researchers contributed to the work, representing Bioluminescence Hub partners such as Brown University, Central Michigan University, U.C. San Diego, the University of California, Los Angeles, and New York University.
Reference: “CaBLAM: a high-contrast bioluminescent Ca2+ indicator derived from an engineered Oplophorus gracilirostris luciferase” by Gerard G. Lambert, Emmanuel L. Crespo, Jeremy Murphy, Kevin L. Turner, Emily Gershowitz, Michaela Cunningham, Daniela Boassa, Selena Luong, Dmitrijs Celinskis, Justine J. Allen, Stephanie Venn, Yunlu Zhu, Mürsel Karadas, Jiakun Chen, Roberta Marisca, Hannah Gelnaw, Daniel K. Nguyen, Junru Hu, Brittany N. Sprecher, Maya O. Tree, Richard Orcutt, Daniel Heydari, Aidan B. Bell, Albertina Torreblanca-Zanca, Ali Hakimi, Tim Czopka, Shy Shoham, Katherine I. Nagel, David Schoppik, Arturo Andrade, Diane Lipscombe, Christopher I. Moore, Ute Hochgeschwender and Nathan C. Shaner, 2 December 2025, Nature Methods.
DOI: 10.1038/s41592-025-02972-0
Funding was provided by the National Institutes of Health, the National Science Foundation, and the Paul G. Allen Family Foundation.
Never miss a breakthrough: Join the SciTechDaily newsletter.
Follow us on Google and Google News.